Talk to your doctor if you develop a new eye condition, experience a sudden decrease in vision, have eye surgery, or develop any eye reactions. Also, talk to your doctor if you’ve ever had increased eye pressure, are taking medication for increased eye pressure, or have risk factors for glaucoma.
LATISSE® may cause increased brown eye coloring (hyperpigmentation). This is rare but is likely permanent if it occurs. Brown eye coloring has occurred infrequently when LATISSE® solution was used. DO NOT APPLY LATISSE® IN YOUR EYE OR TO THE LOWER LID. Ask your doctor for complete application instructions.
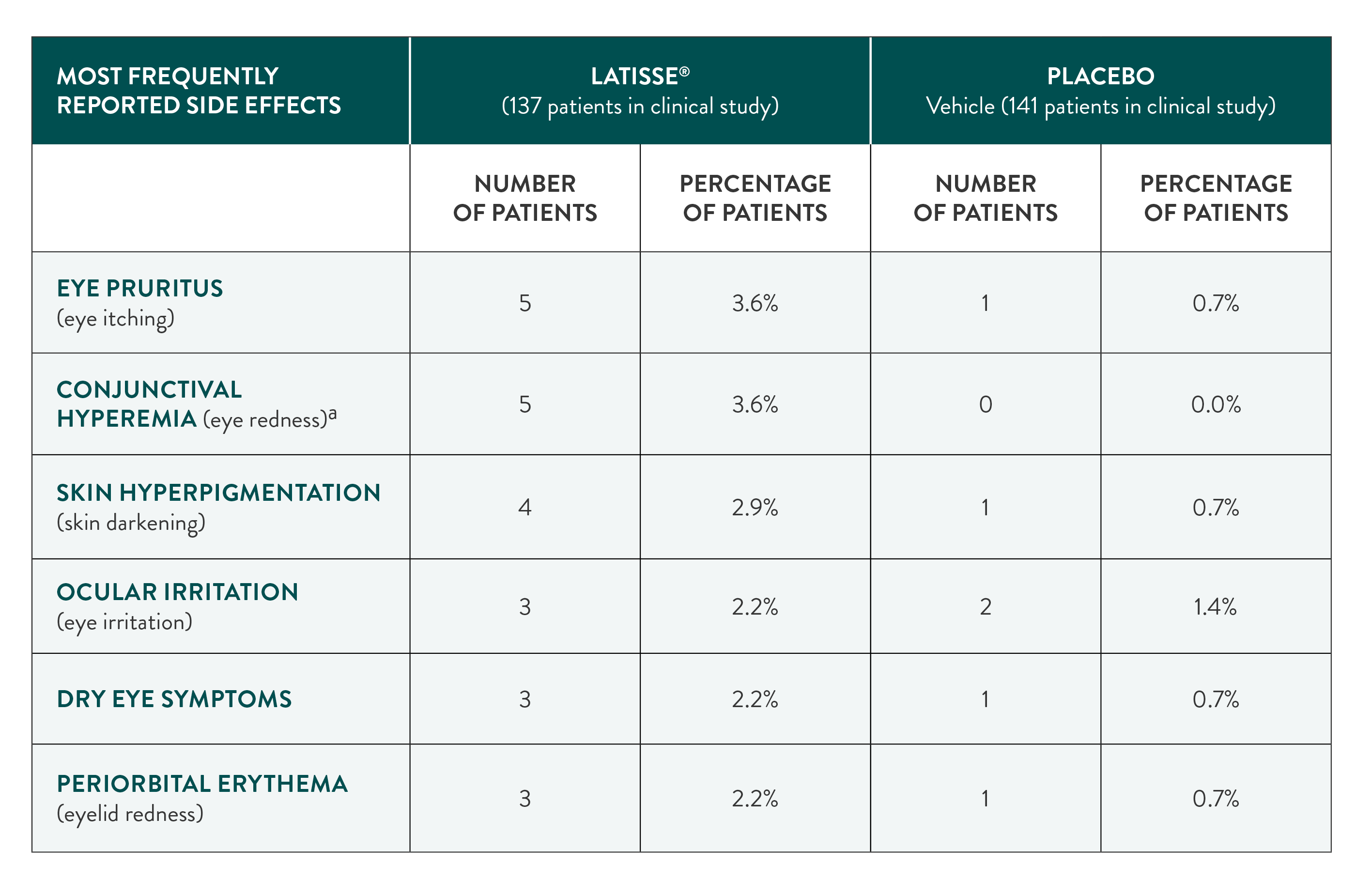
Most frequently reported side effects
in the clinical study
aAll cases of eye redness were fully resolved by the end of the study.
In the clinical study, fewer than 4% of people using LATISSE® (bimatoprost ophthalmic solution) 0.03% experienced these side effects.
This list does not cover all of the possible side effects of LATISSE®.