At 16 weeks
in the clinical study,
lashes were:
106% FULLER
(136 LATISSE® patients) vs 12% for placebo group (140 patients)
25% LONGER
(137 LATISSE® patients) vs 2% for placebo group (141 patients)
18% DARKER
(135 LATISSE® patients) vs 3% for placebo group (138 patients)
WEEK 0
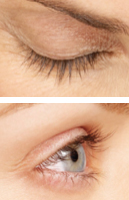
WEEK 4
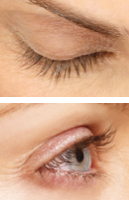
WEEK 8
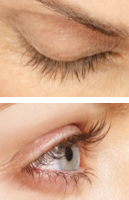
WEEK 12
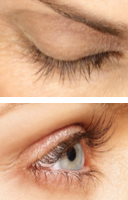
WEEK 16
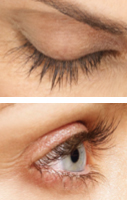
Real patients. Results may vary. Paid users. PRESCRIPTION ONLY. Unretouched, real lashes. No mascara. No extensions. No lash inserts.
*Not statistically significant.

Real patient. Results may vary. Paid user. PRESCRIPTION ONLY.
Unretouched, real lashes. No mascara. No extensions. No lash inserts.


Join Allē today for savings on future treatments
Join AllēEXPAND +
LATISSE® (bimatoprost ophthalmic solution) 0.03% Important Information
Approved UseLATISSE® is an FDA-approved treatment to grow eyelashes for people with inadequate or not enough lashes.
Important Safety InformationDo not use LATISSE® if you are allergic to one of its ingredients. If you use/used prescription products for eye pressure problems, use LATISSE® under doctor care.
LATISSE® (bimatoprost ophthalmic solution) 0.03% Important Information
Approved UseLATISSE® is an FDA-approved treatment to grow eyelashes for people with inadequate or not enough lashes.
Important Safety InformationDo not use LATISSE® if you are allergic to one of its ingredients. If you use/used prescription products for eye pressure problems, use LATISSE® under doctor care. May cause brown darkening of the colored part of the eye which is likely permanent. LATISSE® may cause eyelid skin darkening which may be reversible. Only apply at base of upper lashes. DO NOT APPLY to lower lid. Hair may grow outside the treatment area. If you have eye problems/surgery, consult your doctor. Common side effects include itchy and red eyes. If discontinued, lashes gradually return to previous appearance.
These are not all the possible side effects of LATISSE®. For more information, please talk to your doctor.
Please see LATISSE® full Product Information.





































